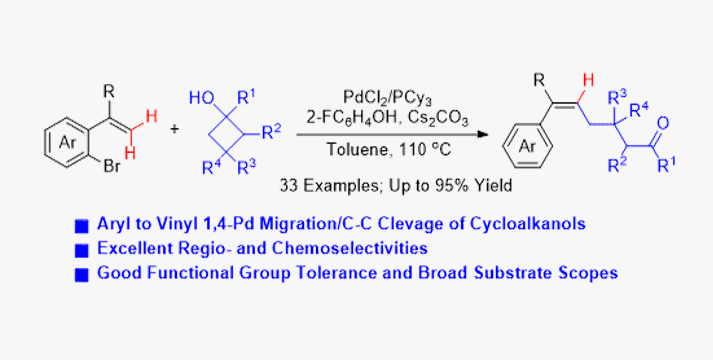
Palladium-catalyzed C−H alkylation of gemdisubstituted ethylenes has been efficiently achieved with cyclobutanols as the coupling partners through an aryl to vinyl 1,4-palladium migration/ring-opening C−C cleavage cascade, giving trisubstituted alkenes in high yields. The protocol features good regioselectivity, high yields, broad substrate scopes, and good functional group tolerance. The mechanistic studies implicate that the cross-coupling reaction occurs via oxidative addition, 1,4-palladium migration, ring-opening C−C cleavage, and reductive elimination. DFT calculations have revealed that the high efficiency of the protocol is attributed to the thermodynamically favored 1,4-palladium migration assisted by 2-fluorophenol.