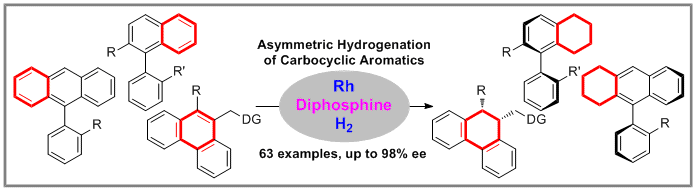
Compared with heteroarenes, homogeneous asymmetric hydrogenation of all-carbon aromatic rings is a longstanding challenge in organic synthesis due to the strong aromaticity and difficult enantioselective control. Herein, we reported the rhodium/diphosphine catalyzed asymmetric hydrogenation of all-carbon aromatic rings, affording a series of axially chiral cyclic compounds with high enantioselectivity through desymmetrization or kinetic resolution. In addition, the central-chiral cyclic compounds were also obtained by asymmetric hydrogenation of phenanthrenes bearing a directing group. The key to success is the introduction of chiral diphosphine ligands with steric hindrance and strong electron-donating property. The axially chiral monophosphine ligands could be obtained by simple conversion of the hydrogenation products bearing the phosphine atom.