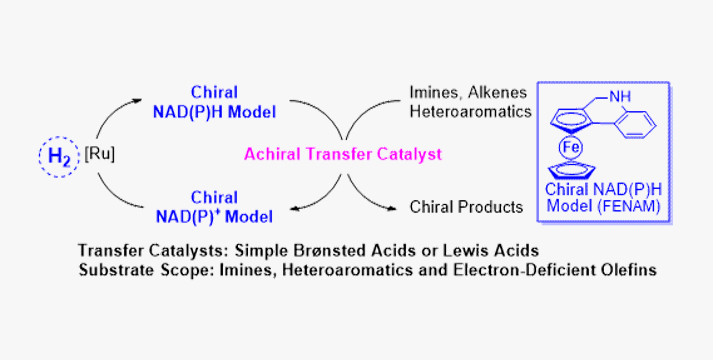
The coenzyme NAD(P)H plays an important role in electron as well as proton transmission in the cell. Thus, a variety of NAD(P)H models have been involved in biomimetic reduction, such as stoichiometric Hantzsch esters and achiral regenerable dihydrophenantheridine. However, the development of a general and new-generation biomimetic asymmetric reduction is still a long-term challenge. Herein, a series of chiral and regenerable NAD(P)H models with central, axial, and planar chiralities have been designed and applied in biomimetic asymmetric reduction using hydrogen gas as a terminal reductant. Combining chiral NAD(P)H models with achiral transfer catalysts such as Brønsted acids and Lewis acids, the substrate scope could be also expanded to imines, heteroaromatics, and electron-deficient tetrasubstituted alkenes with up to 99% yield and 99% enantiomeric excess (ee). The mechanism of chiral regenerable NAD(P)H models was investigated as well. Isotope-labeling reactions indicated that chiral NAD(P)H models were regenerated by the ruthenium complex under hydrogen gas first, and then the hydride of NAD(P)H models was transferred to unsaturated bonds in the presence of transfer catalysts. In addition, density functional theory calculations were also carried out to give further insight into the transition states for the corresponding transfer catalysts.