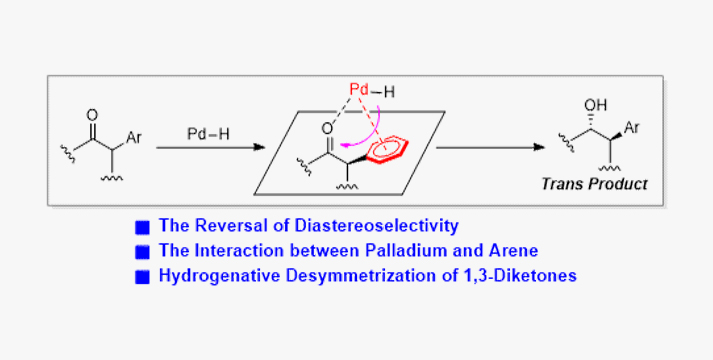
For the metal-catalyzed asymmetric hydrogenation of α-substituted ketones, cis reductive products are generally obtained due to steric hindrance of substituents. Herein, an unprecedented trans reductive products were observed in palladium-catalyzed hydrogenative desymmetrization of cyclic and acyclic 1,3-diketones, providing the chiral trans β-hydroxy ketones with two adjacent stereocenters including one α-tertiary or quaternary stereocenter with high enantioselectivity and diastereoselectivity. Mechanistic studies and DFT calculations suggested that the rarely observed diastereoselectivity reversal is ascribed to the charge-charge interaction between the palladium and aromatic ring of the substrate, which could not only result in the reversal of the diastereoselectivity, but also improve the reactivity.