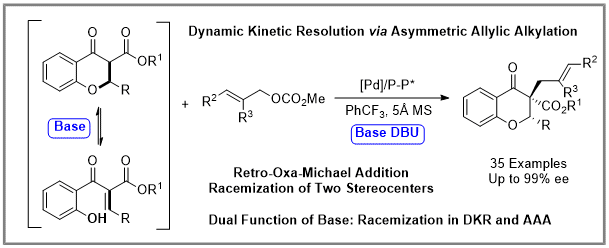
The extension of racemization strategies of dynamic kinetic resolution in organic synthesis is a longstanding challenge, especially racemizing two or more stereogenic centers simultaneously. Through the combination of a palladium-catalyzed asymmetric allylic alkylation and a base-promoted retro-oxa-Michael addition, a dynamic kinetic resolution of 2,3-disubstituted flavonoids was achieved with up to 99% enantioselectivities, and two contiguous stereocenters (including a quaternary stereogenic center) were constructed simultaneously on the nucleophile flavonoids. The key feature of the reaction was a base-promoted retro-oxa-Michael addition for fast racemization of two stereogenic centers on the nucleophiles, which can pave the way to developing asymmetric reactions of flavonoids through dynamic kinetic resolution.