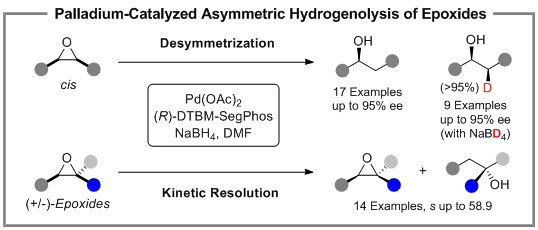
The hydrogenolysis is one of the fundamental transformations in organic synthesis. Despite numerous studies in this field over the last few decades, asymmetric C-O bond hydrogenolysis remains less explored. The enantioselective hydrogenolysis of epoxides catalyzed by transition-metals can usually provide high value-added chiral alcohols. However, β-H elimination and difficulty in stereoselective control are the main challenges. Herein, we successfully realized palladium-catalyzed asymmetric hydrogenolysis of C(sp3)-O bonds of epoxides by strategies including the introduction of borane reagents promoting the fast hydrogen transfer from the borane to the Pd(II)-intermediate and bulky chiral ligands, affecting the reactivity and enantioselectivity. In addition, highly site-specific deuterated alcohols could also be synthesized with readily available sodium borodeuteride as deuterium source. Experimental study and theoretical calculations provided deep insight into the origin of the high enantioselectivity controlled by the sterically demanding biphosphine ligand, and also indicated that the hydrogenolysis undergoes a Pd(0)/Pd(II)-catalyzed process, which may shed light on a strategy for asymmetric transformation of epoxides with transition-metal complexes.